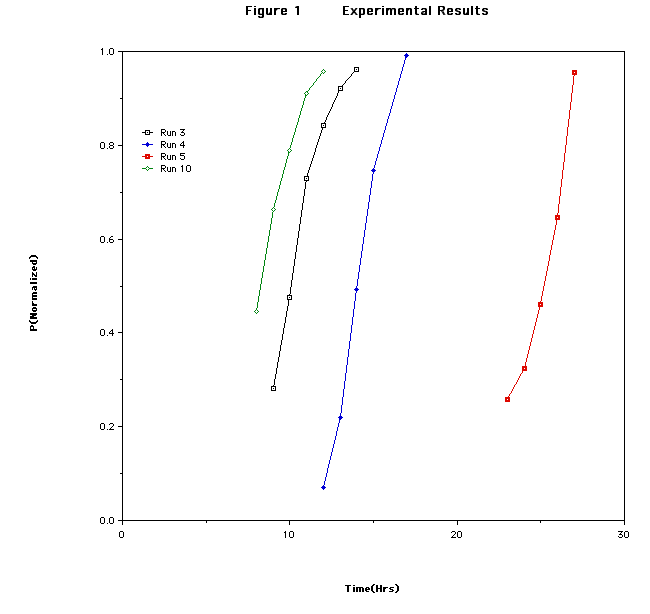
So what do we have here? The first reaction is called Run 3. The reaction appears to do nothing for about 22 hours, then takes off and goes to completion in one hour.
Seeing this for the second run I came into the lab and started taking samples at 12 hours and by this time the reaction had started on its fast rate stage.
This rate profile is called sigmoidal. 'S' shaped.
The graph shows four reaction run results. With increasing number of the reaction experiment, the time for the reaction to start decreases supposedly as a function of how many times a reaction had been run in the flask. I had heard of seasoning the reaction flask and this turns out how seasoning affects the experiment.