Richard Monson discovered that alcohols were dehydrated in refluxing
HMPT(HexaMethylPhosphoricTriamide). Under his direction I started a research project
to elucidate the mechanism of the reaction. At the time Monson thought that the
rate limiting step in the reaction was the elimination step and an experiment was
designed to monitor the reaction, a kinetic experiment. After much false starts
the results obtained indicated to Monson that the rate limiting step was not the
elimination step but the formation of a phosphate ester. Why and how he knew this
was a mystery to me. What I had to show him was a rate profile that was sigmoidal
in shape.
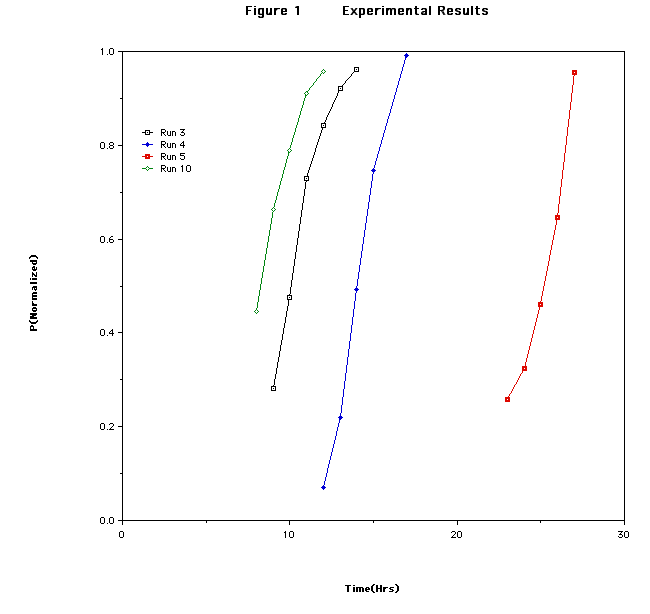
These results were quite puzzling to me. There was nothing in any of the classes
I had taken that had prepared me for a result like this. Originally we thought that
the reaction was second order. Since one of the reactants
was the solvent for the reaction, HMPT, the solvent concentration overwhelms the
concentration of the other reactant, the alcohol, and can be ignored. The system can
thus then be considered pseudo first order, i.e. proportional only to the concentration
of the alcohol. Such a system would be expected to produce a rate profile such as the
following:
Graph of a First Order Chemical Reaction
What Next
