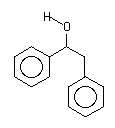
 ==>
==> 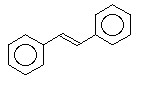
ball and stick model of trans-stilbene
hybridization of carbon
1,2-diphenyl ethanol has two phenyl rings separated by a two carbon chain. The carbons in the rings are sp2 hybridized carbons
when you dehydrate the compound the chain carbons change from sp3 hybridized to sp2 as well
the product trans-stilbene is then fully sp2 hybridized carbon
sp2 hybridized carbon atoms have a free electron so to speak and since there is now a fully conjugated system it absorbs light in the ultraviolet at 292 nm
the property of absorbing light at 292 nm enables the concentration of the chemical to be measured and the analytical method produces numbers
these numbers are what are plotted on the graph shown elsewhere
 ==>
==> 